
31/08/2022: Recruitment to CATHETER II has now closed
03/09/2020: Recruitment to CATHETER II has now restarted at some centres.
16/03/2020: In response to COVID-19, we have paused patient recruitment into this study. We are continuing to follow-up people who are already taking part. It may be that we are in touch with you by a different method than usual (for example we may telephone you to complete questionnaires, rather than send them by post). If you have any questions about the study, please get in touch on 01224 438144 (and leave a message) or email. catheter2@abdn.ac.uk.
If you are a patient and require medical attention or advice, please contact your GP or NHS-111/NHS-24 (by dialling 111).
You can also find information about COVID-19 from the NHS websites in England ,Scotland, Wales and Northern Ireland.
There is also information about COVID-19 on the UK government website.
Urinary catheters are soft tubes inserted into the bladder to drain urine to a collection bag or valve. In the UK, an average of 1 in 1000 people use long-term indwelling catheters (LTCs).
LTCs can be associated with complications such as blockages (where urine accumulates in the urinary bladder and does not drain into the catheter bag), urinary tract infections and urinary incontinence. These complications can impact quality of life and NHS resources.
People with catheters consider catheter blockage as one of the most significant complications of long-term catheter use. Current research tells us that catheter blockages may affect half of all people with LTCs.
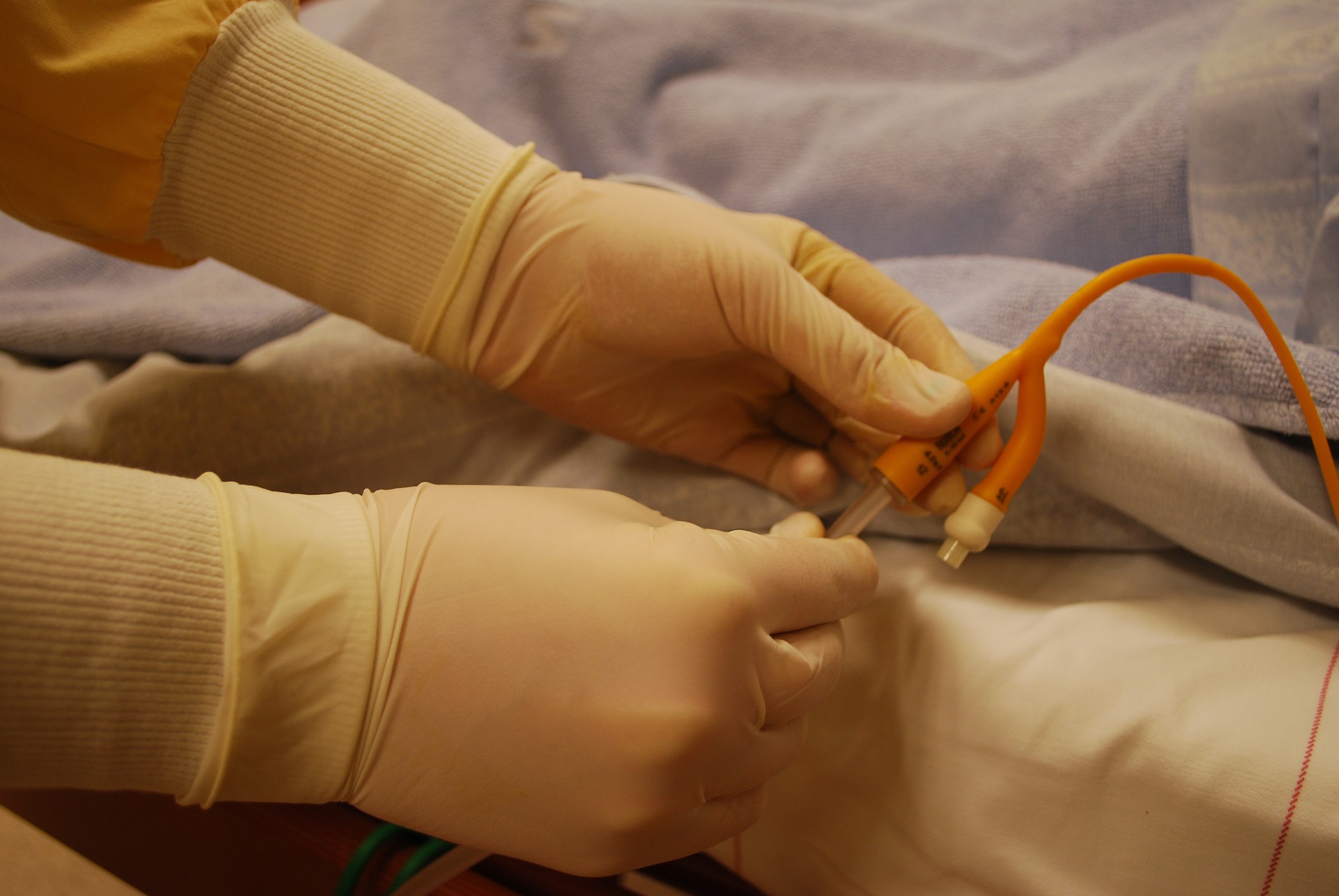
We are recruiting patients at hospitals, GP practices and care homes across the UK. You can see the locations of these in the section of this website “where can I take part”. More locations will be added as the study progresses.
Patients with long-term catheters who are over the age of 18 who are able to undertake catheter washouts or have a designated person to perform washouts are eligible to take part. If you would like to take part or have any questions about the study, you can contact us using the form below or by telephoning 01224 438197 or using the contact us page.

If you are eligible, would like to take part and your local hospital, GP practice or care home is a study site, they will send you information about the CATHETER II study.
To take part you will be asked to sign a consent form and asked some questions about your health and catheter. You will need to provide a small urine sample for a urine dipstick test. After this you will be randomised to receive either:
Whichever group you are in, you will continue to receive the current standard NHS long term catheter care.
If you are in one of the study groups doing the weekly catheter washouts, we will give you the washout solutions free of charge. These will be sent to your home. One of the nurses will train you (and/or one of your family members or carers) how to carry out the washouts correctly.
We will follow you up in the study for 2 years. We will ask you to record any problems with your catheter in a calendar we give you. Each month, a research nurse will contact you by phone and ask about any catheter related problems. Every six months, we will also ask you to complete a questionnaire about your quality of life and your satisfaction with treatment.
There is an extra part of the study that is optional. In this extra part, we will ask some participants to take part in an interview. If you agree to take part in the CATHETER II study, we will ask you whether or not you would like to receive information about these interviews and to be contacted by the interview team.


Most people find flushing their catheter with a washout solution to be a fairly simple procedure. The washout liquid comes in a prefilled sealed bag. At the same time as the routine weekly change of the catheter bag/ valve you connect the washout solution bag to the catheter valve and introduce the washout liquid into the bladder with the help of gravity. You then drain the liquid back into the same bag, again with the help with of gravity.
One of the nurses will train you (and/ or one of your family members or carers) how to carry out the washouts correctly.
In addition, we will give you a detailed instruction leaflet (including illustrations) showing how to carry out the washout.
You can watch a video showing how to administer the saline washout solution here.
You can watch a video showing how to administer the citric acid washout solution here.
Funding acknowledgement
This study is run by the University of Aberdeen, in conjunction NHS Grampian.
This study/project is funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (17/30/02/HTA).
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.