



Multi-centre trials provide key evidence underpinning healthcare practice. However, they invariably take a lot of hard work and expense to complete, with some of this work and expense being devoted to sites that under-recruit. Developing practical methods to identify sites that will recruit to target would be helpful.
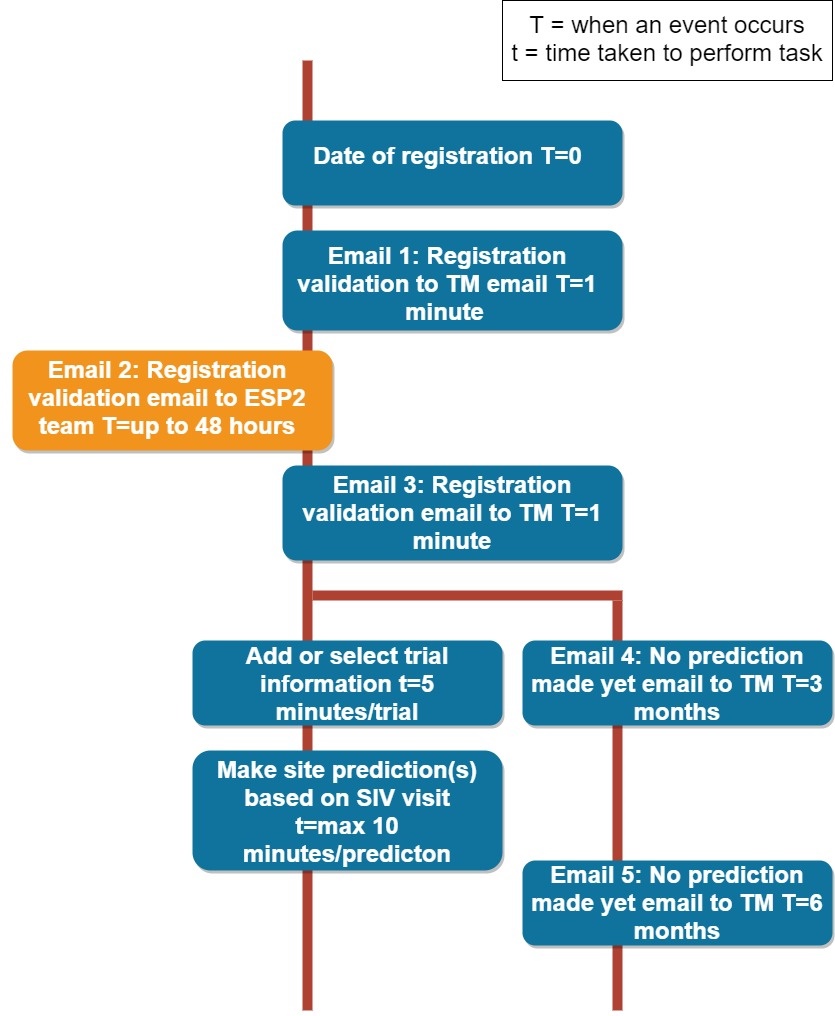
ESP2 aims to evaluate a guided site performance prediction tool for trial managers. The tool was developed during the initial ESP study using practical experience from trial managers. Trial managers were asked to identify reasons why sites might fail to recruit to target in randomised and non-randomised interventional trials.
You can take part in ESP2 if:
If you would like more information about ESP2, including information about taking part, please contact the study team using the contact us link below or email us on ESP2@abdn.ac.uk Or you can just register to take part using the link below.
ESP2 started in 2019 and is expected to run until 2024.

We have decided to suspend ESP2 on 30th April 2022 and to review whether to start again in one year’s time. The pandemic has changed many things, including how trials are run so we want to let things settle and understand the changes before we decide whether to start again in 2023. Thank you for your interest and support of ESP2 so far, we hope to return. The ESP2 study team
Taking part in ESP2 would involve:
For the full details about what is involved please have a look at the Participant Information Leaflet.
We've also prepared some videos about logging your predictions:

Video Guides |
|||
|---|---|---|---|
| To view video on full screen, click on full screen icon in bottom right-hand side of the video | |||
Registering yourself with ESP2: |
|||
Your first log-in after registration: |
|||
Adding a trial to make predictions for: |
|||
Making a prediction: |
|||









Funding acknowledgement
ESP2 is made possible by a collaboration between Trial Forge, the UK Trial Managers’ Network and not least all the Trial Managers who have or will make predictions. We are a bit of a grassroot phenomenon in that it has not been possible to secure project- specific funding but we will not let that stop us (and we do have a bit of funding).
The University of Aberdeen is hosting this study based in the United Kingdom. We will be using information from you in order to undertake this study and will act as the data controller for this study. This means that we are responsible for looking after your information and using it properly. University of Aberdeen/NHS Grampian will keep identifiable information about you for at least 3 years after the study has finished.
Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we have already obtained. To safeguard your rights, we will use the minimum personally-identifiable information possible.
You can find out more about how we use your information https://www.abdn.ac.uk/privacy.
Follow us or visit our social media channel: