


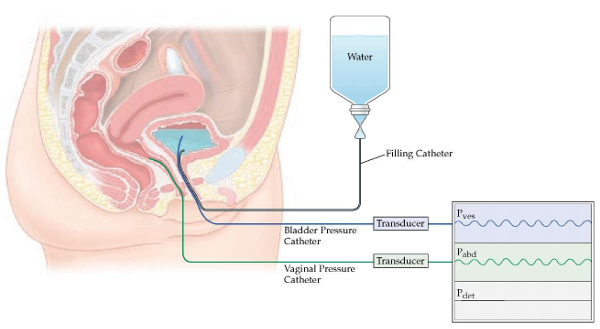
FUTURE is a research study looking at how useful a special bladder test called ‘Urodynamics’ is at improving the treatment results for women affected by refractory overactive bladder (OAB).
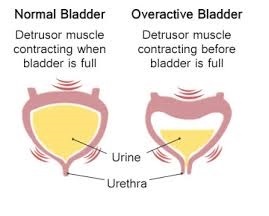
OAB affects 12-14% (12 in every 100 women) of the adult female population in the UK. Symptoms include increased frequency (going to pass urine more frequently); urgency (being unable to hold-on); urgency incontinence (Urinary Leakage being unable to hold-on); and waking up at night to pass urine.
Although rarely life-threatening, OAB can have a considerable negative impact on patients’ quality of life, restricting their social life and ability to work, and up-to social isolation in severe cases.

You may be eligible to take part in FUTURE if:
If you would like to know more about FUTURE, including information about taking part please contact the study office at the University of Aberdeen on 01224 438405 or email future@abdn.ac.uk.
If you are eligible and would like to part in the FUTURE study, you will discuss the study with your consultant as well as the local research nurse. (please see ‘Where can I take part?’ to check if your local hospital is participating).
If, after reading the participant information sheet and receiving satisfactory answers to all your questions, you would like to take part, you will be asked to sign the study consent form and answer some questions about your health.
You will then complete the study questionnaire and bladder diary in the privacy of your own home. After this, your research nurse will randomise you into the study i.e. you will be allocated (in random) to receive Urodynamics or not.
We will follow your progress for 15 months and we will send you the study questionnaire through the post (or email if you prefer) at 3, 6 and 15 months. The questionnaires are returned to the study office in a prepaid envelope which we provide.
You can find out more information on OAB/Urodynamics here: http://www.baus.org.uk/_userfiles/pages/files/Patients/Leaflets/Urodynamics14.pdf
This project was funded by the National Institute for Health Research Health Technology Assessment (HTA) programme and is run by the University of Aberdeen, in conjunction NHS-Grampian. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of National Institute for Health Research, NHS Grampian or University of Aberdeen. Please visit each organisation's website for more information.
University of Aberdeen/NHS Grampian is the sponsor for this study based in the United Kingdom. We will be using information from you and your medical records in order to undertake this study and will act as the data controller for this study. This means that we are responsible for looking after your information and using it properly. University of Aberdeen/NHS Grampian will keep identifiable information about you at least 10 years after the study has finished.
Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we have already obtained. To safeguard your rights, we will use the minimum personally-identifiable information possible.
You can find out more about how we use your information https://www.abdn.ac.uk/privacy.