


In response to COVID-19, we have paused patient recruitment into this study. We are continuing to follow-up people who are already taking part. It may be that we are in touch with you by a different method than usual (for example we may telephone you to complete questionnaires, rather than send them by post). If you have any questions about the study, please get in touch on 01224 438084 (and leave a message) or email. raaceno@abdn.ac.uk.
If you are a patient and require medical attention or advice, please contact your GP or NHS-111/NHS-24 (by dialling 111).
You can also find information about COVID-19 from the NHS websites in England ,Scotland, Wales and Northern Ireland.
There is also information about COVID-19 on the UK government website.
RAACENO (pronounced "ra-chino") is a study that will investigate whether adding exhaled nitric oxide measurements to standard asthma care will help prevent asthma attacks in children. We all breathe out a gas called nitric oxide. We can measure exhaled nitric oxide using a special breathing device. People with asthma breathe out more nitric oxide than people without asthma because nitric oxide is produced by the allergic cells which are present in the lungs of people with asthma. We know that these allergic cells build up before an asthma attack. We now want to see whether adding exhaled nitric oxide measurements to standard asthma care will help prevent asthma attacks in children.

We are recruiting patients at hospitals in the UK. You can see the location of these hospitals in the section of this website "where can I take part". Children and young people in the catchment area for these hospitals can take part if they
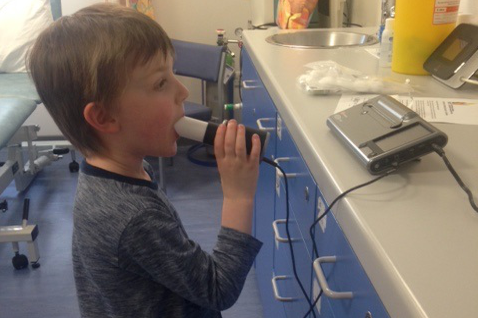
We all have a gas called nitric oxide in the air we breathe out. People of all ages with asthma have more nitric oxide in their breath than people without asthma. Nitric oxide levels go up before and during an asthma attack and come back down after an attack. We can measure nitric oxide levels in breathed out air. In this study, we will investigate whether adding nitric oxide measurements to standard care for children with asthma can help reduce asthma attacks.
We will recruit 502 children aged 6-15 years with a diagnosis of asthma, who currently use inhaled corticosteroids, and who have had an asthma exacerbation in the previous 12 months.

We will randomise children to either:
All children taking part will have a meter on their inhaled steroid inhaler. This will allow the research team to know how much of the inhaler is being taken.
The primary outcome is asthma exacerbation (attack) which will be assessed at the 3, 6, 9 and 12 month follow-up visits, and confirmed with data held in medical records. Secondary outcomes include time to first exacerbation, number of exacerbations, and quality of life.
The study includes a health economic evaluation; data will be collected on asthma-related health care use, and time off school and away from other activities.
Around 200 children will also be invited to take part in optional mechanistic studies alongside the main study. The optional mechanistic studies involve:
Around 20 children will be invited to take part in an interview at the end of the 12 month follow-up to explore attitudes to, and acceptability of the FeNO measurements. Five research nurses will also be interviewed about the feasibility of FeNO measurements.



To see if asthma attacks can be reduced in children by measuring exhaled nitric oxide and using these measurements to help guide asthma treatment.
Nitric oxide is a gas that we all have in our breath and people with asthma breathe out more nitric oxide than people without asthma. Nitric oxide does several jobs in our bodies including controlling the blood flow through blood vessels and helping our brains remember things. In addition to being a helpful thing, it is also produced by allergic cells rather like a car produces fumes. Nitric oxide does not smell, and we can't see it, but we can measure it.
Our work leading up to this study suggests that using exhaled nitric oxide levels to help guide asthma treatment might reduce asthma attacks in children with asthma. We are doing this study to find out whether this is the case or not.
We will be asking children and young persons aged between 6 and 15 years to take part. They also need to be under the care of a hospital doctor for their asthma, to be on inhaled preventer medicine (called inhaled corticosteroids) and to have had an asthma attack treated with steroid tablets in the past year. We will be recruiting children in approximately 25 hospitals around the UK.
Children and young people taking part will be in the study for 12 months. The whole study will last 4 years.
If we find that asthma attacks are reduced with the help of the nitric oxide measurements we will recommend that all children with a recent asthma attack have nitric oxide testing.
This all depends on what we find in this study. If we find that asthma attacks are reduced by using nitric oxide measurements to help guide treatment, we will do a similar study in children and young people in general practices to see if nitric oxide can reduce asthma attacks there too.
This study was funded by the Efficacy and Mechanism Evaluation (EME) Programme of the National Institute for Health Research (NIHR). Please visit the NIHR website for more information: www.nihr.ac.uk.
University of Aberdeen/NHS Grampian is the sponsor for this study based in the United Kingdom. We will be using information from you and your medical records in order to undertake this study and will act as the data controller for this study. This means that we are responsible for looking after your information and using it properly. University of Aberdeen/NHS Grampian will keep identifiable information about you at least 10 years after the study has finished.
Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we have already obtained. To safeguard your rights, we will use the minimum personally-identifiable information possible.
You can find out more about how we use your information https://www.abdn.ac.uk/privacy.