


Endometriosis is a common condition which affects one in ten women from puberty to menopause. It occurs when cells similar to those lining the womb grow outside it, generally within the pelvis. These cells behave like the cells lining the womb, causing internal bleeding at the time of periods, scarring and pain.
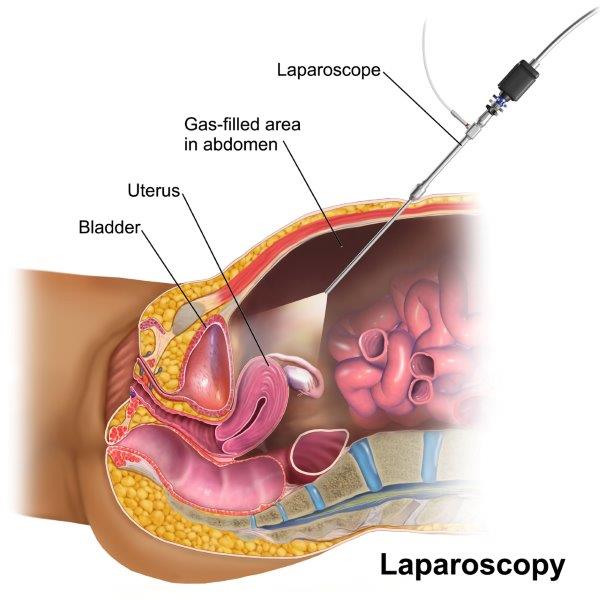
Endometriosis is diagnosed by laparoscopy (keyhole surgery) which identifies areas of endometriosis which can then be destroyed or removed. However, surgery rarely provides lasting relief and pain can return in up to half of treated women within five years. To reduce the chance of regrowth of endometriosis and recurrence of pain, women who are not trying to get pregnant are offered the combined oral contraceptive pill or other contraceptives containing hormones called progestogens.
You may be eligible to take part in REGAL if:
• you are female and aged between 21-49 years old
• you have recurrent pain following laparoscopic treatment for endometriosis
• you wish to avoid removal of your ovaries and a hysterectomy
• your doctor considers you suitable for both study arms (Gonadotrophin Releasing hormone analogue (GnRHa with HRT versus further laparoscopic surgery)
Please see ‘Where can I take part?’ to check if your local hospital is participating.

If you would like to know more about REGAL, including information about taking part, please contact the study office at the University of Aberdeen on 01224 438196 or email regal@abdn.ac.uk
If you are eligible and wish to participate in REGAL and your hospital is a recruiting site, you will receive further information from your local research team.
To take part you will be asked to sign the study consent form and complete a questionnaire about your pain and quality of life. After this, the local research nurse will randomise you into the study i.e. you will be allocated (at random) to receive one of the treatments below:
• Gonadotrophin Releasing hormone analogue (GnRHa) with HRT
• Further laparoscopic surgery
We will regularly follow your progress for 24 months.
We will send you the study questionnaire through the post (or email if you prefer) at 6, 12, 18 and 24 months from the date you entered the study. The questionnaires are returned to the study office in a prepaid envelope which we provide.
Funding acknowledgement
The project is funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (reference NIHR127280) and run by the University of Aberdeen, in conjunction with NHS Grampian.
The views expressed are those of the author(s) and do not necessarily those of the NHS, the NIHR or the Department of Health & Social Care.
Participant Information
University of Aberdeen/NHS Grampian is the sponsor for this study based in the United Kingdom. We will be using information from you and your medical records in order to undertake this study and will act as the data controller for this study. This means that we are responsible for looking after your information and using it properly. University of Aberdeen/NHS Grampian will keep identifiable information about you at least 25 years after the study has finished.
Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we have already obtained. To safeguard your rights, we will use the minimum personally-identifiable information possible.
You can find out more about how we use your information here :
https://www.abdn.ac.uk/about/privacy/research-participants-938.php or by contacting the Data Protection office at dpa@abdn.ac.uk